Commercial production of indigenous vaccine developed by ICAR that provides complete protection against LSD to start in 4-5 months
The commercial production of the indigenous Lumpi-ProVacInd vaccine developed by ICAR that provides 100 per cent protection against Lumpy Skin Disease (LSD) is likely to start in the next four to five months. Agri Innovate, a DARE company, has issued the EoI document for the commercialization of the vaccine. Once the agreements are signed with the manufacturers and the process of statutory approval necessary before commercial production gets completed, the production of the vaccine will start and the vaccine will be available to immunize the cattle.
The commercial production of the indigenous Lumpi-ProVacInd vaccine developed by the Indian Council of Agricultural Research (ICAR) that provides 100 per cent protection against Lumpy Skin Disease (LSD) is likely to start in the next four to five months. This information was shared by Dr Bhupendra Nath Tripathi, Deputy Director-General (Animal Science), ICAR, in an interview with Rural Voice. He said that Agri Innovate, a Department of Agricultural Research and Education (DARE) company, had issued the Expression of Interest (EoI) document for the commercialization of the vaccine. Some of the animal vaccine manufacturers in the country have shown interest in this. Once the agreements are signed with the manufacturers and the process of statutory approval necessary before commercial production gets completed, the production of the vaccine will start and the vaccine will be available to immunize the cattle.
Dr Tripathi said that the vaccine provided 100 per cent production against LSD in cattle and used live virus. They started working on the vaccine at the end of 2019 and trials were done on the cattle in July 2022 that have been fully successful. The vaccine has been jointly developed by the ICAR’s National Research Centre on Equines (NRCE) at Hisar, Haryana, and the Indian Veterinary Research Institute (IVRI) at Izatnagar, Bareilly, Uttar Pradesh.
Said Dr Tripathi, “It (Lumpi-ProVacInd vaccine) is also homologous, providing 100 per cent protection against LSD in cattle. Currently, we are only administering goat pox and sheep pox virus vaccines. These are heterologous vaccines offering only cross-protection (up to 60-70 per cent) for cattle against LSD, by virtue of all the three viruses belonging to the same capripoxvirus genus.”
He says that Lumpi-ProVacInd vaccine remains safe for a long time. The vaccines developed for Covid-19 have only 5-6 months’ durability but this is not the case with Lumpi-ProVacInd vaccine. Besides, this vaccine does not have to be kept in freezing conditions. It needs only refrigerator temperature (4-8 degrees Celsius). Which will make its use easy. This has been possible due to the presence of live virus in this vaccine.
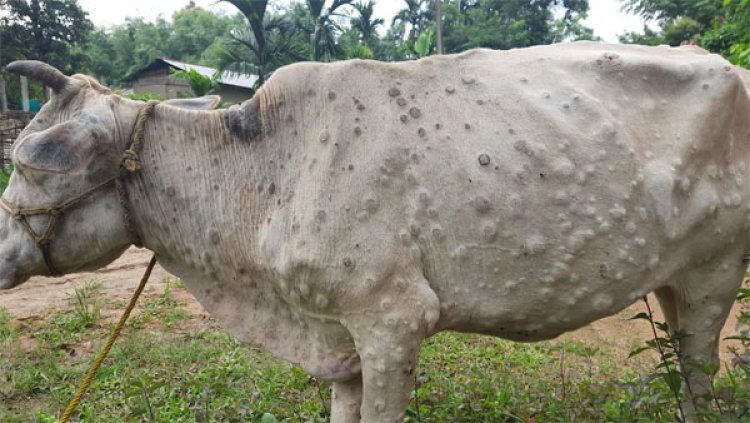
Rural Voice gave information in a September 2 story that more than 1.1mn cattle had been affected by LSD and about 50,000 killed up to August 31. In view of this crisis, the vaccine developed to protect against this disease will prove very effective. You may click on the link given below to know in detail about the number of cattle affected and what the government thinks.
The development of this vaccine has an interesting story. Scientists continued to work on it even through the Covid-19 period. Work started on the vaccine when LSD first broke out in Odisha in 2019. According to Dr Tripathi, this vaccine was developed under the leadership of Navin Kumar, a virologist and Principal Scientist at NRCE.
Work started on the vaccine at NRCE, Hisar, in December 2019 after taking a sample of the LSD virus. The virus was then isolated and gene-sequenced. It was then cultured. The culturing was done over 50 generations (“passages”) and took about 17 months. The pathogenicity loss started around the 30th passage. “We did the sequencing of the virus genome at the start and the 10th, 30th and 50th passages. The attenuated live virus was identified as a vaccine candidate after the 50th passage and testing it on our laboratory mice and rabbits,” informed Kumar. This process was carried out at the Mukteshwar centre of IVRI. Dr Tripathi says that this is a live virus and it grows very fast. This is why the vaccine has a reduced cost.
Experimental trials of the vaccine candidate on the natural host (cattle) were undertaken at IVRI in April 2022. While 10 calves were vaccinated, five were kept along with them without being vaccinated. It takes about a month from the time of vaccination for full immunity to develop. After one month, both sets of calves were injected with the virulent virus. The control calves showed most of the LSD symptoms, whereas the vaccinated animals had developed full immunity.
Since July 2022, field trials have also taken place. The trials were done at Banswara, Jodhpur, Udaipur and Alwar in Rajasthan, Mathura in Uttar Pradesh and Hansi and Hisar in Haryana. The vaccinated cattle were found to be fully protected against LSD while others got affected by the disease.
Regarding the commercial production of the vaccine, officials say that in order to meet the required level of production, several manufacturers will have to produce the vaccine at the same time. Dr Tripathi says that going by the cattle population of the country, about 200mn doses of vaccine are required. It will not be possible to bring the disease to a complete end unless 80 per cent of the cattle are vaccinated.
There are six veterinary vaccine manufacturers in the country. These include Indian Immunologicals Ltd, Hester Biosciences, Brilliant Bio Pharma, MSD Animal Health and Biovet Private Ltd. Also, public sector companies in a few states manufacture veterinary vaccines. Several vaccine manufacturers have expressed interest in the EoI issued by Agri Innovate. The manufacturers will be provided the technical know-how and the requisite materials after an agreement with this company. But after this, these manufacturers will have to go through a similar statutory approval process as do the human-vaccine manufacturers. The process will be similar to the release of the Covid vaccine.
An official from the Ministry of Animal Husbandry and Dairying told Rural Voice that the government would provide help in the approval process to the companies that come forward for the commercial production of the vaccine. The said official expressed hope that in view of the scale on which the vaccine is required, several companies would come forward to manufacture the vaccine.



 Join the RuralVoice whatsapp group
Join the RuralVoice whatsapp group









































